The annual average concentrations of (a) PM2.5, (b) MDA8 O3, (c) NO2,... | Download Scientific Diagram

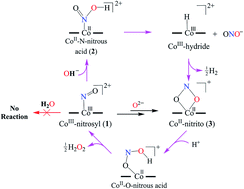
In this reaction, does NO2+ act like a Lewis acid, Lewis base, Bronsted acid or Bronsted base? A. Lewis Acid B. Lewis Base C. Bronsted Acid D. Bronsted Base E. Lewis Acid
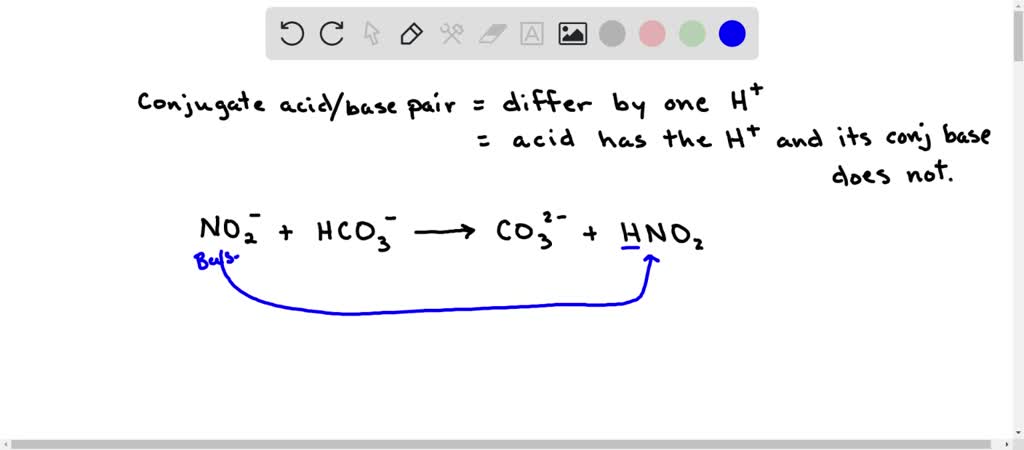
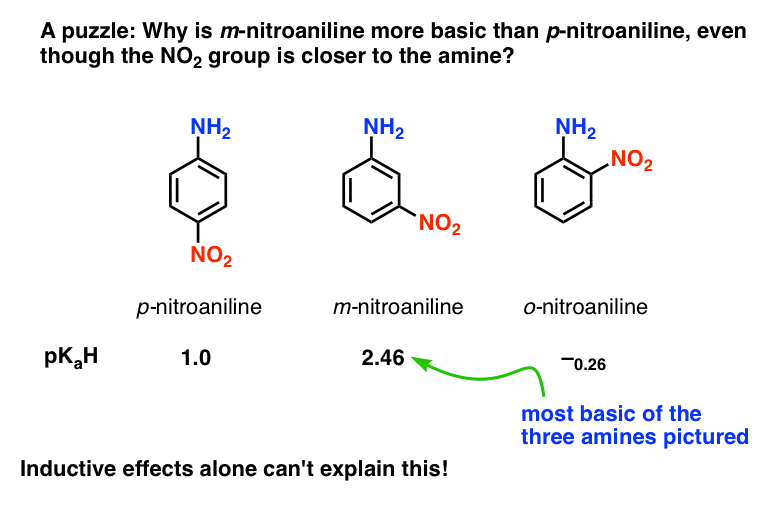
SOLVED: Consider the following reaction: NO2 - + HCO3 - ⇌ CO3 2- + HNO2 Identify the acid, base, conjugate acid and conjugate base.

Buy Golden Brass Candle Stand with Chapta Emboss Design on Base/Decorative Item for Home,Mandir,Church,Birthday Party and The Best Item to Gift.No2. Height 2.5 inch Online at Low Prices in India - Amazon.in